

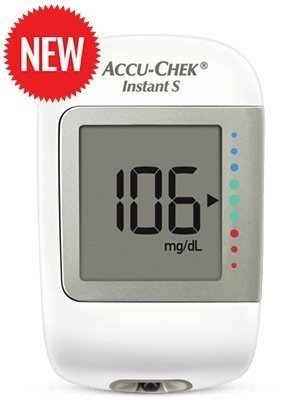
Accu-Chek Instant S BLOOD GLUCOSE MONITORING SYSTEM WITH 1 VIAL OF 10 TEST STRIPS – Fast Shipping to USA & Canada
- Demonstrated precision, adheres to the standards of ISO 15197:2013/effortless preparation with no setup necessary.
- Immediate understanding with the target range indicator.
- Memory capacity: A minimum of 720 BG and 30 control measurements are retained in the device, with only the most recent result visible on the display.
- Time measurement: Under 4 seconds. Battery duration: 750 tests. Includes 10 Lancet needles.
- Stability of test strips: 18 months following the production date.
Free Delivery on 3 Quantities or 3 Products
Delivered in 8–17 days
Get $5 off purchases of $60 or more — enter coupon code ('XMAX2025' ) at checkout.
Every order includes a FREE surprise Christmas gift...
Get 7% OFF on all future orders for 1 year — Christmas offer, today only!
Free Delivery on 3 Quantities or 3 Products
Delivered in 8-17 days
Get $5 off purchases of $60 or more — enter coupon code ('XMAX2025' ) at checkout.
Every order includes a FREE surprise Christmas gift...
Get 7% OFF on all future orders for 1 year — Christmas offer, today only!
Order help?
Connect with us on WhatsApp – personal support, not a chatbot.
Guaranteed Safe Checkout

Features & Compatibility
The Accu-Chek Instant S blood glucose monitoring system reduces testing obstacles by enhancing user-friendliness through its various components and features. It includes a TRI tool and USB connectivity to the Accu-Chek Connect online platform.
Additional information
| Ship to countries |
United States, Canada |
|---|---|
| Brand |
Accu-Chek |
| Sold By |
Yamuna Desi Store |
| weight |
150 g |









Wood –
Good choice for routine checks.
Bethany –
Excellent starter kit for instant results.
Avery Robinson –
Iowa – Fast shipping! Order reached me sooner than expected.
Isabella Rodriguez –
Alabama – Package arrived very fast! Excellent service.
Vihaan Deshmukh –
Alberta ensured fast and efficient delivery; order arrived safely.
Henry –
Fast and consistent performance.
Daniel –
Handy device, simple operation.
Weber –
Includes everything to start testing.
Christopher –
Great display and result speed.
Henry –
Strips work without issue.